
Students examine graphs showing how enzymes and substrates interact. Enzymes are biological catalysts that accelerate chemical reactions by lowering the activation energy required for the reaction to occur. In most cases, the rate of an enzyme-catalyzed reaction increases with an increase in substrate concentration.
The graphic shows a traditional lock-and-key model of enzyme and substrate interactions. The shape of the enzyme fits to the shape of the substrate, which initiates the reaction. This model explains the specificity of enzyme-substrate interactions. For example, the lactase enzyme is specific to the lactose protein.
Initially, when the substrate concentration is low, the enzyme’s active sites are not fully occupied. As more substrate molecules are available, they can easily bind to the active sites, leading to an increase in the rate of the reaction.
As the substrate concentration increases, the rate of the reaction also increases. At a certain point, the enzyme’s active sites become nearly saturated with substrate molecules. At this stage, the enzyme is functioning at its maximum capacity. Other factors can affect the rate of reaction, such as temperature and pH.
Enzymes and Substrates Worksheet
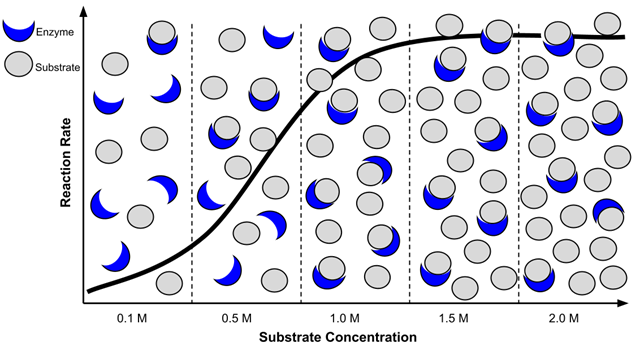
Students examine a graphic that illustrates how enzymes and substrates interact. They count the number of available molecules at each concentration. Analysis reveals that the reaction rate is fast at first, but levels off when the substrate is saturated.
In the second section, students also look at the number of products created over time. Once the enzyme has run out of substrates to interact with, the reaction stops.
Students can also learn about these processes in this enzyme coloring activity. I generally cover the basics of enzymes in both freshman biology and AP Biology. AP Biology does additional investigations with enzymes, such as exploring yeast and catalase reactions.

