Have you ever noticed that when you put hydrogen peroxide on a wound, it bubbles? The bubbles form from an reaction where hydrogen peroxide is broken down into water and oxygen. This reaction is a fantastic way to observe enzymes and substrates in action.
I have several activities exploring this reaction. In one, students simply observe bubbles when hydrogen peroxide comes into contact with different tissues. This enzyme investigation requires minimal set-up and is appropriate even for beginning students.
For my AP Biology students, I have used a floating disk method to obtain quantitative data. Though, this method can be problematic. Sometimes the disks don’t float. Sometimes they float so quickly that it can be difficult to accurately measure time.
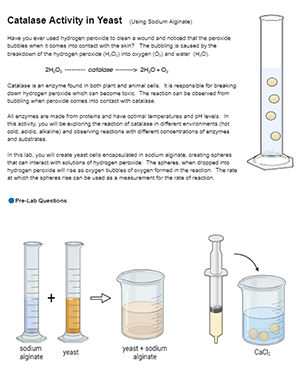
In this version of the lab, students use sodium alginate to create spheres of yeast. The procedure is fairly simple and students can make the spheres and store them for several days. Then, they drop the spheres into hydrogen peroxide and measure how quickly they float to the surface. The time it takes to reach the surface is an indirect measure of the speed of the reaction. Students observe the spheres in different concentrations of hydrogen peroxide (3%, 1.5%, and .75%).
Once students have the procedure mastered, they explore how temperate affects the rate of reaction. Students design the experiment, gather data, and write a lab report explaining the results. Students can even do this lab before they have a complete understanding of enzymes and substrates. The introduction gives them enough background information to explore the phenomenon.
This is the set-up preparation for my class. I put the yeast and sodium alginate in medicine cups (10 ml of each). Then students make the spheres using the large pipettes. Students make the spheres and we store them for data collection the following day.


