Metabolism: Energy & Enzymes
Energy = the capacity to do work or produce heat
First Law of Thermodynamics:
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed in an isolated system. Instead, it can only change forms or be transferred from one object to another or between different forms of energy within the system.
Second Law of Thermodynamics
In a closed system, the overall entropy (a measure of disorder or randomness) tends to increase over time.
Why is energy an important theme in biology?
-
Metabolism: Organisms obtain energy through metabolic processes such as cellular respiration (breaking down food to release energy) or photosynthesis (converting light energy into chemical energy). These processes involve the transformation and utilization of energy-rich molecules like glucose.
-
Homeostasis: Energy is essential for maintaining internal balance and stability within organisms. Cells use energy to regulate temperature, pH levels, and other physiological conditions necessary for life.
-
Movement and Activity: Energy powers movement at various levels, from the beating of a heart to the migration of animals. Muscles use energy to contract, allowing organisms to move and perform various activities.
-
Growth and Development: Energy is crucial for growth and the development of organisms. It's utilized in the synthesis of new molecules, cell division, and the repair and maintenance of tissues.
-
Reproduction: Energy is required for the reproductive processes in organisms, from the production of gametes (sperm and egg cells) to supporting the growth and development of offspring.
Metabolic Pathways & Enzymes
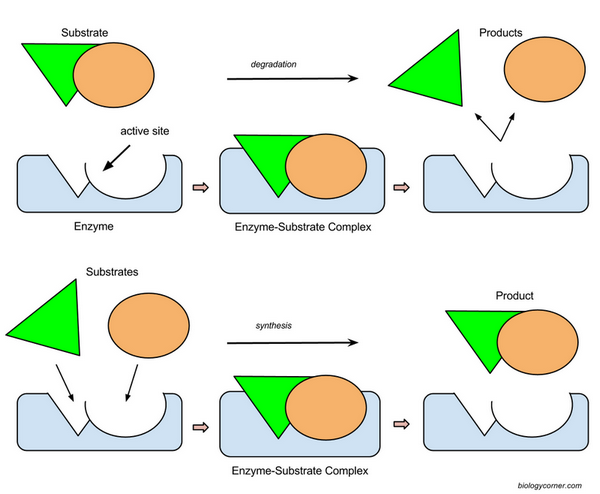
Enzyme - protein molecule that functions
as a catalyst to speed reactions by lowering activation energy
Substrate - reactants in the enzymatic reaction, this is what an enzyme attaches
to.
Examples of Enzymes
| Enzyme | Action | Where |
| Lactose | Lactose (milk) to simple sugars | Small intestine |
| Amylase | Starch to simple sugars | Saliva |
| Pepsin | Proteins to amino acids | Stomach |
| Lipase | Fats to fatty acids | Pancreas / Stomach |
| Alcohol dehydrogenase | Alcohol to sugar | Liver / Stomach |
Properties of Enzymes:
Enzymes are made of proteins.
They speed up chemical reactions
They remain unchanged after each reaction and can therefore be reused
Each enzyme is specific for a substrate
Induced Fit Model- substrates and enzymes fit together like a lock and key.
Factors Affecting Enzymatic Speed
1. Substrate concentration
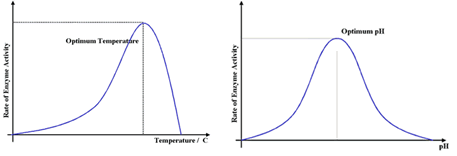
2. Temperature & pH *
3. Enzyme concentration
Enzymes can be denatured - they change shape so much that they are no longer effective. High temp or pH can cause denaturation.
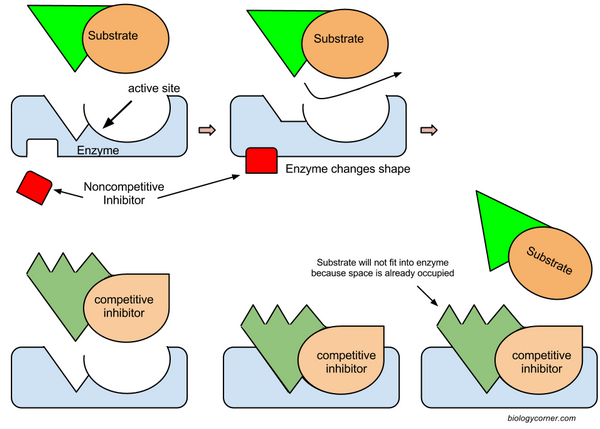
Enzymatic Inhibition -
Competitive Inhibition and Noncompetitive Inhibition
**Both are forms of feedback inhibition
Some inhibitors are NOT reversible - poisons like cyanide, lead poisoning all affect enzymes
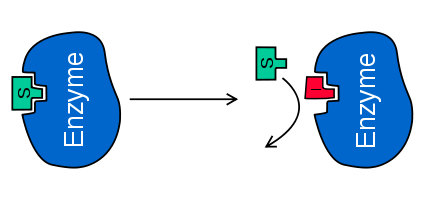
QUESTION: What type of inhibition is pictured below? (Answer: Competitive Inhibition)

Enzymes in Action
- Yeasts break down sugar (glucose)
- Siamese cats have an enzyme that works at lower temperatures only, causing the nose and ears to become a darker color than the rest of the body.
Enzyme Worksheets
Enzyme and Substrate Concentration (Graph Analysis)
Exploring Enzymes and Inhibition
Enzyme Labs
Catalase Activity in Yeast Using Sodium Alginate