McMush Lab

Objective: Students will use indicator solutions to test for biological macromolecules found in a Happy Meal.
Background Information:
Cells are made up of small molecules like water, ions such as sodium and magnesium, and large carbon-based molecules. There are four important types of large carbon-based molecules in living organisms- proteins, carbohydrates (sugars & starches), lipids (fats), and nucleic acids. Proteins, carbohydrates, and fats serve as nutrients in the food that we eat.
Nucleic acids are made of monomers called nucleotides that make up the information storing molecules in our cells, DNA. While we consume DNA when we eat other plants and animals, the DNA from our cells is copied each time our cells divide, it is not something that we must consume in our diets, though the building blocks to make new DNA is synthesized from materials we do consume. In this activity, we will not be testing for the presence of nucleic acids.
Carbohydrates are used by living organisms as an important source of energy. Simple carbohydrates are made of carbon, hydrogen, and oxygen atoms in a 1:2:1 ratio. This ratio means that for every one carbon atom present in the carbohydrate, there are two hydrogen atoms and one oxygen atom present. The monomers of carbohydrates are referred to as monosaccharides. Common examples of monosaccharides include glucose, fructose, galactose, ribose, and deoxyribose.
Sucrose, or table sugar, and lactose, the sugar found in milk, are double sugars made from two monosaccharides. Benedict’s Solution can be used to test for the presence of a monosaccharide, like glucose. In the presence of glucose, Benedict’s Solution turns from blue to orange when heated (orange is positive, blue is negative).
When many monosaccharides join together, the resulting molecule is called a polysaccharide. Important polysaccharides include cellulose, starch, and chitin. Lugol’s Solution can be used to test for the presence of a polysaccharide, like starch. In the presence of starch, Lugol’s Solution turns from amber to dark blue (dark blue is positive, amber is negative).

Lipids are also made of carbon, hydrogen, and oxygen but the ratio of carbon, hydrogen, and oxygen atoms is not 1:2:1. Instead, lipids have a much greater number of carbon and hydrogen atoms with few oxygen atoms present. their individual subunits (monomers) are fatty acids and glycerol. Lipids are organic compounds that are do not dissolve in water, examples include fats, oils, and the wax covering leaves.
The nonpolar bonds that form between the carbon and hydrogen atoms of a lipid cause them to be hydrophobic or “water repellent” molecules, as opposed to hydrophilic or “water loving” molecules. This attribute explains why water and oil do not mix. Sudan III Solution can be used to test for the presence of a lipid. In the presence of a lipid-rich solution and water, Sudan III Solution forms a distinct layer or clump in the well or test tube (layers or clumps is positive, no layers or clumps is negative).
The large number of carbon to hydrogen bonds also serves to make lipids energy-rich storage molecules. One gram of a lipid stores twice as much energy as one gram of carbohydrate or protein. Lipids from animals are referred to as fats and are solid at room temperature, whereas those found in plants are referred to as oils and are liquid at room temperature. Fats and oils are triglycerides, which are biomolecules that are composed of a glycerol and three fatty acids.
One important relative of triglycerides are phospholipids. Phospholipids differ in structure from regular triglycerides in that phospholipids are made of a glycerol and two fatty acids. A charged phosphate group replaces the third fatty acid. The arrangement causes phospholipid molecules to have both hydrophilicand hydrophobic regions. This feature also makes phospholipids an ideal structural component of cell membranes.
Proteins are made of monomers called amino acids, which are composed of atoms of carbon, hydrogen, oxygen, and nitrogen. Proteins are large and complex molecules that combine to form various components of living organisms such as muscle fibers, enzymes, and hemoglobin. Proteins are made from specific sequences of amino acids. A string of amino acid monomers joined together by peptide bonds is called a polypeptide.
Biuret’s Solution (Cu2SO4 + NaOH) can be used to test for the presence of protein. In the presence of protein, Biuret’s Solution turns from blue to violet (violet is positive, blue is negative).
Prelab Summary: After reading the information above, complete the following tables.
Biological Macromolecule |
Monomer(s) |
Function |
Food Sources |
Examples |
Carbohydrates |
|
|||
Proteins |
|
|||
Nucleic Acids |
|
None |
||
Lipids |
|
Macromolecule |
Test Reagent |
Positive Test Color |
Negative Test Color |
|
|
||
|
|
||
|
|
||
|
|
Question: Which macromolecules are present in the McMush food slurry?
Hypothesis: If carbohydrates, proteins, and lipids are present in the food slurry, then each will test positive in the presence of their respective reagents.
Materials: beaker, hot plate, well plate, tongs, test tubes, test tube rack, McMush food slurry, glucose control, starch control, protein control (egg solution), lipid control (oil), distilled water, Benedict’s Solution, Lugol’s Iodine Solution, Biuret’s Solution, Sudan Solution
Safety: Goggles must be worn. Long hair should be pulled back.
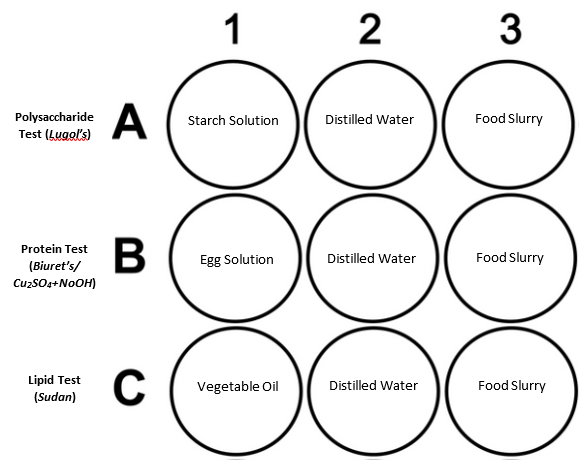
Procedure/Data Collection: Use the well plate image below as a guide for setting up your well plate. Record the color of the test in the box in each well on the well plate image.
Test |
Directions |
Monosaccharide |
Place ~2 ml each of glucose solution, distilled water, and the food slurry you are testing in separate test tubes. Add 5 drops of Benedict's solution to each test tube. Mix by holding the tube between the thumb and index finger of one hand and gently thumping the tube. |
Polysaccharide |
To view the results easier, place the well plate on a white sheet of paper. Place ~2 ml each of starch solution, distilled water, and the food slurry in separate wells on the well plate (be careful not to mix up the droppers). Use a clean dropper to add 3 drops of Lugol’s Iodine Solution to each well. Observe and record the results in the Data Table. |
Protein |
Place ~2 ml each of egg solution, distilled water, and the food slurry in separate wells on the well plate. Slowly add about 10 drops of the Biuret’s solution to each well and mix carefully. Observe and record the results in the Data Table. |
Lipid |
Place ~2 ml each of vegetable oil, distilled water, and the food slurry in separate wells on the well plate. Add 5 drops of Sudan III Solution to each well. Observe and record the results in the Data Table. |
Data Collection:
1. Record the results from the monosaccharide test demo below:
Monosaccharide |
Glucose Solution |
Water |
Food Slurry |
|
2. Use well plates to conduct the tests for the remaining macromolecules. Record any color changes or observations on the well plate chart.
Data Analysis:
1. What is the purpose of rows 1 & 2 on the well plates?
2. Make a Claim that summarizes which macromolecules were present in the food slurry in this experiment.
3. Using data collected from this experiment, provide Evidence that supports the claim.
4. Using background knowledge and data from this lab, provide Reasoning that uses the evidence to justify the claim.
Teacher Tips:
McMush Slurry - place the entire contents of a happy meal, including a white (non-diet) soda into a blender or apple juice. Add about 250 ml of distilled water and distribute the slurry in test tubes or other containers. I used clean dixie cups because I suspected that some students might taste it. I’ve kept this overnight in the refrigerator for make-ups, but it needed to be remixed.
Starch solution can be made with water and cornstarch.
Be sure to use glucose for the Benedict’s test. Table sugar (sucrose) will not result in a color change. Glucose can be stored in powdered form for a long time..
Egg solution can be made with egg whites or gelatin

